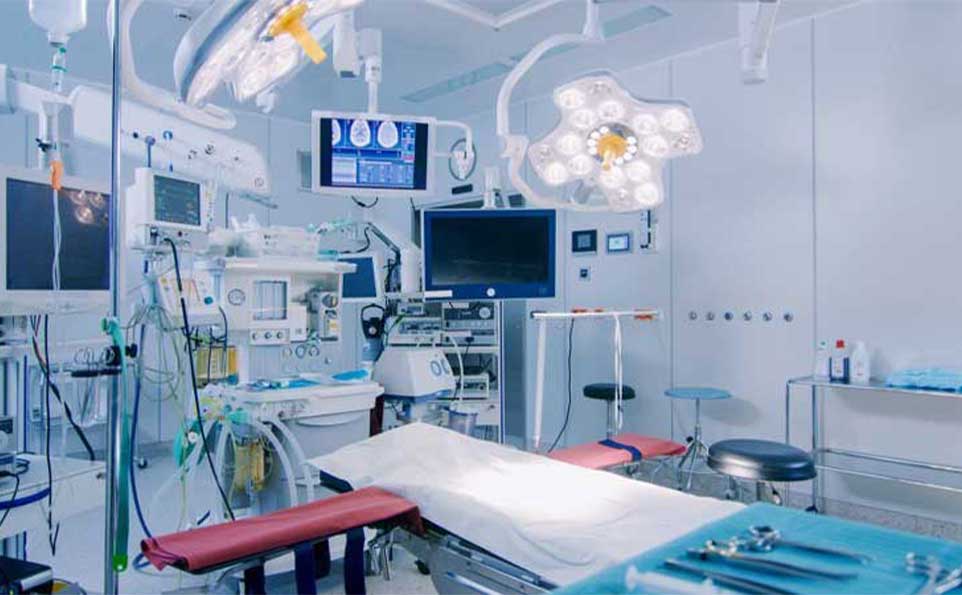
Medical devices are the unsung heroes of the healthcare industry. From the simplest syringes to complex imaging machines, these devices are instrumental in diagnosing, treating, and improving the lives of countless patients worldwide. At the core of every reliable and effective medical device lies a commitment to precision and quality in production. In this blog, we'll explore how precision and quality are the lifeblood of medical device production, and why they matter so much.
Precision Engineering
Medical device production begins with meticulous planning and design. Engineers delve into the intricacies of each device, carefully considering every component, mechanism, and function. Precision is not just a buzzword; it's a fundamental requirement. Every millimeter and microgram matters, especially in devices where a slight error could have life-altering consequences.
The tolerances in medical device manufacturing are incredibly tight. Components must fit together flawlessly, and moving parts must operate with minimal friction. Achieving this level of precision requires cutting-edge technology, such as CNC (computer numerical control) machining, laser cutting, and 3D printing. These tools enable manufacturers to create parts with exacting specifications, ensuring the device's reliability and accuracy.
Quality Assurance at Every Step
Quality is non-negotiable in the medical device industry. From raw materials to the finished product, rigorous quality control measures are in place at every step of the manufacturing process. This includes inspections, testing, and documentation to verify that each device meets the highest standards of safety and performance.
Materials used in medical devices must be carefully chosen to ensure biocompatibility, durability, and resistance to sterilization. For example, surgical instruments are often made from stainless steel due to its corrosion resistance and ability to withstand repeated sterilization. These materials are subject to strict quality checks to ensure they meet the necessary standards.
Compliance with Regulations
The medical device industry is heavily regulated to protect patient safety. Manufacturers must navigate a complex web of regulations and standards imposed by health authorities like the FDA in the United States or the CE Marking in Europe. Compliance with these regulations is paramount, and manufacturers invest significant time and resources to ensure their devices meet all requirements.
Testing and Validation
Before a medical device ever reaches a healthcare professional's hands, it undergoes extensive testing and validation. This includes performance testing, sterilization validation, biocompatibility testing, and more. Each test is designed to verify that the device functions as intended and poses no risk to patients.
Innovation and Continuous Improvement
Precision and quality are not static goals in medical device production; they are part of a continuous journey of improvement. As technology evolves, manufacturers seek innovative ways to enhance device performance, reduce size, improve usability, and increase durability. This drive for innovation has led to remarkable advancements in medical devices, making healthcare more efficient and effective.
Conclusion
In the world of healthcare, precision and quality are the heart and soul of medical device production. These principles are what make medical devices trustworthy and safe, and they are the foundation upon which patient care relies. As technology continues to advance, the commitment to precision and quality will remain unwavering, ensuring that medical devices continue to play a pivotal role in improving and saving lives around the world.
 Hospital Furniture
Hospital Furniture Medical Devices
Medical Devices MSR Products
MSR Products Office Furniture
Office Furniture